Quality policy
Mission Statement
At ERA Sciences, we are focused on the delivery of practical and realistic high-quality solutions for clients, predominantly in the Life Sciences, Medtech and Biotech sectors to support their data reliability needs across the GxP data lifecycle. Solutions may include services and support, training and/or products developed for a specific client or wider identified industry need.
The reliability and integrity of data is fundamental to quality committed organizations. Getting data integrity right ensures the manufacture and provision of safe, reliable and effective medicines and products.
Understanding the implications of poor data practices on patients, products and compliance with regulatory authority requirements is what we know, and how to anticipate data risks and mitigate appropriately is a core competency that we provide to all clients.
We have been in operation since February 2021, with our registered business address in Meath, Ireland and operating from our principal place of business Suite 913, The Greenway, Ardilaun Court, Court C, 112-114 St Stephen’s Green, Dublin, D02TD28, Ireland.
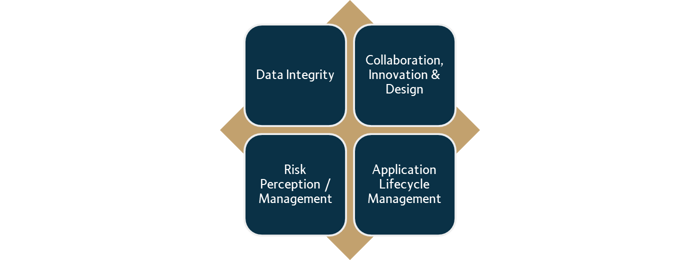
Our company recognizes four data reliability excellence pillars Figure 1 and is committed to providing quality solutions in these key areas:
-
Data Integrity
-
Stakeholder Engagement
-
Risk Perception/ Management
-
Application Lifecycle Management
Our employees are highly skilled with exceptional industry experience and individually and collectively strive for the best professional client outcomes in a well-organized and quality focused manner. We continually upskill and stay informed of current and emerging regulatory expectations including:
-
US FDA 21 CFR Part 11 for Electronic Records and Signatures
-
EU EudraLex Volume 4 Annex 11 for Computerised Systems
-
Expected changes to regulations including Adoption of CSA Approaches, Annex 11 Updates, AI Governance
We apply a risk-based approach during client solutioning and are familiar with and informed by GAMP Ed 2 and Agile methodologies and ICH Q9 R1.
Our core company values of integrity, passion for excellence and willingness to innovate enable us to provide high quality data reliability solutions for clients that leverage best in class technologies when and wherever possible. Our work environment encourages each employee to perform his or her role to the best of their ability whilst encouraging both personal and professional growth opportunities.
We have adopted a largely remote working model to accommodate employee needs whilst having no deleterious impact on clients. We leverage a variety of cloud-based solutions for all business, quality management and internal training needs. All quality activities are formally documented in our Quality Management System (QMS) which we will continue to develop in 2024.
We provide training to clients in a variety of formats including self-directed and virtual instructor led events on our learning management platform https://eflex.erasciences.com/ and site based as required. We recognize that learning is a journey, and clients should choose the ‘size’ that best fits them. This could mean face-to-face classroom training, online self-paced eLearning or instructor interactive e-Modules.
An expert team develops and delivers high quality training in response to emerging client needs.
Client site visits for training and support activities are available as required where we follow all required client site procedures. Additionally in person meetings and solutioning workshops are accommodated at our Dublin business office.
As a company we engage with industry events and forums and welcome opportunities to speak or contribute our expertise to improve awareness of data reliability enablers and challenges. Industry events also allow us to upskill across broader data reliability topic ranges and identify opportunities for further growth including product development.
Our Commitments
To understand client data pain points across the data lifecycle and examine where efforts and resources are optimally focused in order to drive best client outcomes associated with data reliability and regulatory compliance
-
To investigate and develop high quality product solutions that will enhance client data reliability and integrity
-
To continual organizational improvement through the implementation of quality standards and international best practices
-
To apply an agile methodology to our internal practices ensuring we focus our resources appropriately and can respond to new or changing client needs in an effective manner
-
To provide transparency for clients on in-progress and completed activities ensuring their business goals are/have been achieved - our client scorecards are leveraged across many industry sectors
-
Setting appropriate goals and objectives relating to safety, health and quality for all employees
Acronyms and frequently used terms
|
Acronym/ |
|
|---|---|
|
AI |
Artificial Intelligence -the ability of a machine to display human-like capabilities such as reasoning, learning, planning and creativity |
|
CFR |
The Code of Federal Regulations is the codification of the general and permanent rules published in the US Federal Register by the departments and agencies of the US Federal Government. Title 21 of the CFR is reserved for rules of the Food and Drug Administration |
|
CSA |
Computer Software Assurance is a new way of looking at the traditional Computer System Validation (CSV) approach through application of critical thinking and consideration of risk whereby the focus is on what matters – patient safety, product quality and data integrity |
|
EudraLex |
EudraLex is the collection of rules and regulations governing medicinal products in the European Union |
|
FDA |
US Food and Drug Administration |
|
GAMP |
Good automated manufacturing practice is a set of guidelines for manufacturers and other automation users to maintain operational efficiency and reliability GAMP is also a subcommittee of the International Society for Pharmaceutical Engineering (ISPE) |
|
GAMP Ed 2 |
GAMP 5 Guide 2nd Edition provides a structured framework for planning, executing, and documenting the validation process, emphasizing the importance of a risk-based approach and ongoing maintenance |
|
GxP |
GxP is a general abbreviation for the "good practice" quality guidelines and regulations |
|
ICH |
International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use |
|
ICH Q9 R1 |
The R1 revision is intended to provide guidance on quality risk management principles and tools that can be applied to different aspects of pharmaceutical quality |
|
PIC/s |
Pharmaceutical Inspection Co-operation Scheme |
-
Change History
|
Version |
Summary of Changes |
|
4 |
Inclusion of emerging regulatory expectations Addition of Terms Table |
|
3 |
Document updated to include ERA Sciences learning management system eFlex platform Document updated to communicate Dublin business address for face-to-face activities where indicated as a client requirement Version 2 document imported from original ERA Sciences controlled repository to EDMS |
|
2 |
Correction of Date for ERA Sciences in operation April 2021 to February 2021 |
|
1 |
New Document signed using DocuSign |
Last updated: 02 Nov 2023