Hurray! We applaud the new release of guidance around risk management to the life sciences with ICH Q9 Revision 1: https://ich.org/page/quality-guidelines
After careful deliberation over a process that begun in 2021 and lasted a pandemic, this guidance represents a welcome increase in risk management maturity for high-reliability organisations in the industry. Well done to all in the expert working group who contributed to this final guidance being released.
I'm sure you're interested to know what the changes are and what they mean to empower your organisation. We have attached a redline of the changes below, and here are the highlights:
-
It is now recognised that subjectivity can directly impact risk assessment outputs. There is an entire section (5.3) in ICH Q9 R1
-
Product availability risks are a consideration by regulators and will be a focus. In addition to manufacturing oversight it is important to have effective oversight of supply chain partners. In addition to 'supply chain' in the responsibilities section, there is an entire section (6.1) in ICH Q9 R1 (and in Annex II.9).
-
Appropriate risk-based decision-making addresses uncertainty through the use of knowledge and helps assure product quality. It is important to be explicit in upfront decisions that inform the risk approach going forward. Risk-based decisions cannot justify a practice that would be deemed unacceptable by regulators or guidance. There is an entire section (5.2) in ICH Q9 R1
-
An understanding of formality can lead to efficient resource utilisation and better risk-based decision-making with a focus on high risk issues. There is an entire section (5.1) in ICH Q9 R1
-
Risk review is expected to be more proactive, seeking to inform current risk-based decisions when scientifically robust knowledge emerges
-
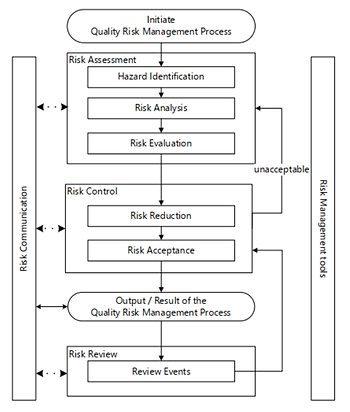
Hazard identification reframes the conversation to separate hazards from risks, an increase in maturity for the industry. This change is reflected on the Quality Risk Management diagram.
To see a complete redline of the changes since the first ICH Q9 in 2005, check out this document:
We have been working on risk training specifically around subjectivity and formality and its impact to data integrity and computerised systems to align with this new guidance. If this is of interest to you reach out to me at andy.oconnor@erasciences.com or keep an eye on our learning platform:
Good luck on your risk journey!


Comments